Oxidative Phosphorylation Part 1:
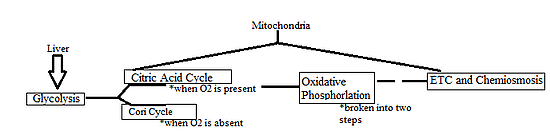
Oxidative Phosphorylation (OP) follows the Citric Acid Cycle and is broken down into two components: the Electron Transport Chain (ETC) and Chemiosmosis. For this section, we will only be discussing the ETC and its mechanisms.
Electron Transport Chain
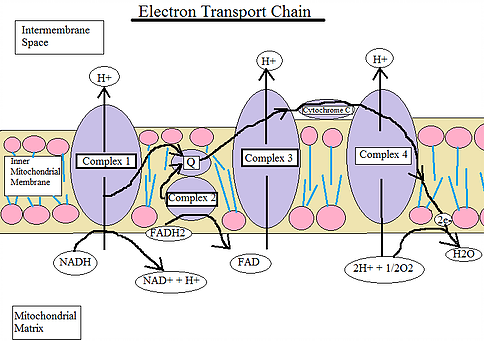
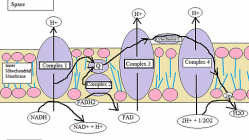
The ETC is located in the mitochondria of eukaryotic cells and is used for extracting energy via a series of reactions and transfers from oxidation of sugars. *In short, the ETC moves from one electron donor to the next electron acceptor by redox reactions. There are four complexes that create the ETC.
Complex 1: NADH Coenzyme Q Reductase or NADH Dehydrogenase
Complex 2: Succinate Dehydrogenase or Succinate-Coenzyme Q Reductase
*The only enzyme that participates in both the Citric Acid Cycle and ETC
Complex 3: Coenzyme Q Reductase
Complex 4: Cytochrome C Reductase
Further Examination of Complex Mechanisms:
Complex 1: The first complex in the ETC, formally known as NADH Coenzyme Q Reductase, is named such because this protein complex will transfer two electrons from NADH to Coenzyme Q
**This complex contains an FMN Prosthetic Group which is absolutely necessary for its activity; therefore this complex is a flavoprotein.
In this complex, the binding of NADH and its transfer of two electrons in the form of a hydride to FMN creates NAD+ and FMNH2 (the subsequent steps involve the transfer of electrons one at a time to a series of iron-sulfur complexes). The last step of this complex is the transferring of two electrons one at a time to Coenzyme Q (CoQ). CoQ, like FMN and FAD, can function as a two-electron donor/acceptor and as a one-electron donor/acceptor. CoQ is a mobile electron carrier because its isoprenoid tail makes it extremely hydrophobic and lipophilic. If diffuses freely in the bilipid layer of the inner mitochondrial membrane.
In short, Complex 1 of the ETC establishes the hydrogen ion gradient by pumping four hydrogen ions across the membrane from the matrix into the inner membrane space.
Complex 2: In the second complex of the ETC, FADH2 (which bypasses Complex 1) is reduced and delivers electrons directly to the ETC. During these processes, we first see a Ubiquinone (Q molecule), this molecule connects the first and second complex to the third complex.
*The Ubiquinone is lipid soluble and freely moves through the hydrophobic core of the membrane.
Here, the Ubiquinone is reduced to QH2 and delivers its electrons to the next complex.
*The Ubiquinone receives the electrons from NADH from Complex 1 alongside the electrons from FADH2 from Complex 2 (including the enzyme Succinate Dehydrogenase). This enzyme and FADH2 forma small complex that delivers electrons directly to the ETC, which again, bypass Complex 1. Since these electrons bypass the proton pump in Complex 1, meaning they do not energize, fewer ATP molecules are made from the FADH2 electrons.
The number of ATP molecules obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex 3: In the third complex of the ETC, protons are pumped through the membrane and pass its protons to Cytochrome C for transport to the fourth complex of proteins and enzymes.
*Cytochrome proteins have a prosthetic heme group (the heme molecule is similar to the heme in hemoglobin however it carries electrons, not oxygen). Because of this, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe2+ (oxidized) and Fe3+ (reduced).
**The heme molecules in the cytochromes have slightly distinctive characteristics due to the effects of the different proteins binding them (making each complex).
Complex 4: In the fourth complex of the ETC, oxygen is reduced and then picks up two hydrogen ions from the surrounding medium to make water.
*The fourth complex is comprised of Cytochrome proteins C, A, and A3; it also has two heme group cones in each of the Cytochromes and three copper ions.
The removal of the hydrogen ions from the system also contributes to the ion gradient used in the process of Chemiosmosis.
Terms Defined:
Complex: A structure consisting of a central atom, molecule, or protein weakly connected to surrounding atoms, molecules, or proteins.
Prosthetic Group: A non-protein group forming part of or combined with a protein
FMN Prosthetic Group: Non-peptide compound of FMN that is a phosphoric ester (meaning without water) of riboflavin and acts as a prosthetic group for many flavoproteins
Flavoprotein: An enzyme containing riboflavin and linked chemically with a protein; active in the oxidation of foods in animal cells (eukaryotes)
Riboflavin: Vitamin B complex necessary for carbohydrate metabolism and growth; comes from ribose
Ribose: A pentose sugar obtained by the hydrolysis of RNA
*RNA is more unstable than DNA because RNA contains ribose sugars rather than deoxyribose sugars
Ubiquinone: A lipid soluble substance that is a component of the ETC and accepts electrons from Complex 1 and 2 and delivers them to Complex 3; any class of compounds that act as electron-transfer agents in cellular respiration (substituted quinones)
Quinone: Another term for 1,4-benzoquinone
Isoprenoid Tail: Made of a hydrocarbon called isoprene; isoprene is a branched-chain unsaturated hydrocarbon (meaning it contains one or more double bonds between carbon atoms)
Hydrocarbon: A compound of hydrogen and carbon
Cytochrome C: A small hemeprotein found to be associated with the inner membrane of the mitochondrion; they are iron containing hemeproteins central to which heme groups that are primarily responsible for the generation of ATP via the ETC